Gray Treefrog
Dryophytes versicolor
Male Aggression Call
Common Name: |
Gray Treefrog |
Scientific Name: |
Dryophytes versicolor |
Etymology: |
|
Genus: |
Dryophytes The name "Dryophytes" is derived from the Greek words "dryos," meaning tree, and "phytes," meaning plant, which refers to the arboreal habits of these frogs. |
Species: |
versicolor is derived from the Latin words versi which means "various" and color which means "color". |
Average Length: |
1.3 - 2 in. (3.2 - 5.1 cm) |
Virginia Record Length: |
|
Record length: |
2.4 in. (6 cm) |
*Note: Our two native gray treefrogs are identical in appearance. In the field the only two ways to distinguish D. chrysoscelis from D. versicolor is by their call and in some cases geographic location.
Cope's Gray Treefrogs (Dryophytes chrysoscelis) and Gray Treefrogs (Dryophytes versicolor) are members of a cryptic, diploid-tetraploid species complex. This has resulted in considerable taxonomic confusion, especially in early reports. As a result of this confusion, many authors have chosen to combine the descriptions and distributions of these species. In this account, I have attempted to separate, as much as possible, the literature related to D. chrysoscelis and D. versicolor. For categories where data are lacking or where there is a question regarding the species identification, data from the sister species are reported. This approach is at least partially validated by the genetic analysis of this complex by Ptacek et al. (1994) and the growth and development studies of Ptacek (1996).
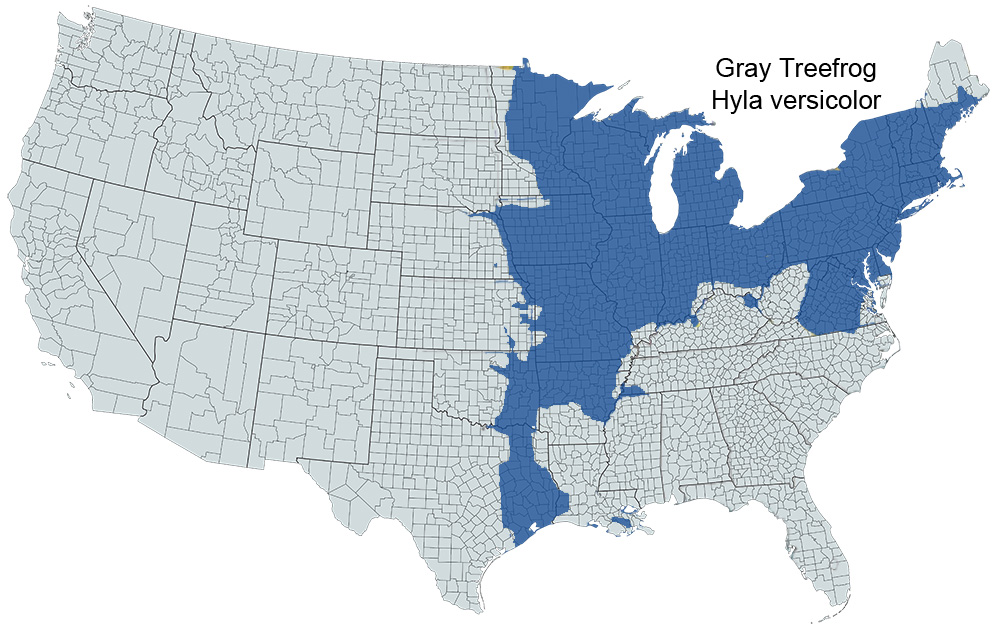
Historical versus Current Distribution - Because of the cryptic nature of Cope’s gray Treefrogs (Dryophytes chrysoscelis) and Gray Treefrogs (Dryophytes versicolor), discussions of these species are nearly always intertwined. Dryophytes versicolor was originally described by LeConte (1825). Wright and Wright (1949) list Cope's Gray Treefrogs as subspecies of Gray Treefrogs. Fitzgerald et al. (1981) reviewed the early taxonomy of this complex. The distribution of Cope's Gray Treefrog has long been associated, and usually combined, with that of Gray Treefrogs. Noble and Hassler (1936) reported two call types along the East Coast of the United States. A fast-trilling, harsh call type was found in the southern United States, while a slower trilling, more mellow sounding call type was found at higher latitudes. The two call types coexisted in a railroad yard in Baltimore. Wright and Wright (1949), apparently not realizing the importance of the call differences, restricted the distribution of D. chrysoscelis to east-central Texas, east into southern Arkansas and northwestern Louisiana. Blair (1958a) reported distinct geographic distributions for two call types of what was then considered D. versicolor. Fouquette and Johnson (1960) demonstrated call discrimination by females of both call types. Recent depictions show the distribution of the Dryophytes chrysoscelis/versicolor complex to encompass much of the eastern United States, including the eastern Great Plains (eastern portions of Nebraska, Kansas, Oklahoma, and eastern Texas), Minnesota south to Louisiana, and east of the Mississippi River to the Atlantic coastline, excluding northern Maine and peninsular Florida, south of the panhandle (Conant and Collins, 1998).
Species identification has long been problematic in this group. Noble and Hassler (1936) first reported discrete call types along a latitudinal cline. Blair (1958a) plotted distinct geographic distributions of two call types across the range of what was then considered D. versicolor. Johnson (1959, 1961, 1963, 1966) reported genetic incompatibility between the call types, prompting him to designate the faster trilling call type as D. chrysoscelis and the slower trilling call type as D. versicolor. The diploid–tetraploid nature of this complex was first suggested when Wasserman (1970) reported that D. versicolor was a tetraploid (4N = 48). Later, Bogart and Wasserman (1972) confirmed the diploid (2N = 24) nature of D. chrysoscelis. Ralin (1977a) discussed call variation between D. chrysoscelis and D. versicolor along an east–west transect from central Texas to Louisiana. Gerhardt (1974a), Ralin (1977a), and Cline (1990) quantified call differences between eastern and western races of D. chrysoscelis. Bachmann and Bogart (1975), Cash and Bogart (1978), and Green (1980) demonstrated partial discrimination between the two species measuring cell diameter. Later, Ralin and Rogers (1979) reported that D. versicolor were morphologically and genetically (Ralin and Selander, 1979) intermediate between eastern and western populations of D. chrysoscelis. Cline (1990) combined acoustical, morphological, and genetic analyses in central Oklahoma, Kansas, Missouri, and Arkansas. Cline (1990, using Ralin, 1977a) shows Cope's Gray Treefrogs in east-central Texas (excluding the eastern 1/5 of the state), north into central Oklahoma (excluding the eastern 1/4 of the state), eastern Kansas, extreme eastern Nebraska, extreme eastern South Dakota, extreme eastern North Dakota, western and southern Minnesota, Iowa, extreme southwest Wisconsin, most of Missouri, east in a band across south-central Illinois, much of central Indiana, and much of western and central Ohio, south through most of Arkansas and Louisiana, Mississippi, southwestern Tennessee, Alabama, the Florida Panhandle, Georgia, South Carolina, eastern North Carolina, southeastern Virginia, and southeastern Maryland. Dryophytes versicolor is divided into two groups. A southwestern group extends from east-central Texas north through central and eastern Oklahoma and western Arkansas into eastern Kansas and southwestern Missouri. A northern and eastern group extends north from roughly southern New Jersey through central Missouri, and south along the Appalachian Mountains, possibly as far south as Alabama and Georgia. Ralin and Selander (1979) reported isozyme differences between eastern and western forms of D. chrysoscelis. Gerhardt (personal communication) has narrowed the gap between northern and southern D. versicolor groups.
Numerous attempts have been made to identify species and determine distribution of these cryptic species at the state level. Hoffman (1946, Virginia), Walker (1946, Ohio), Brown and Brown (1972, Illinois), Bogart and Jaslow (1979, Michigan), Matson (1988, Ohio), Little (1983, and Little et al., 1989, Ohio/West Virginia), Johnson (1987, Missouri) used call characteristics. Jaslow and Vogt (1977, Wisconsin), Hillis et al. (1987, Kansas), Chapin and Trauth (1987, Arkansas) used histological techniques. Recent research at the state level has begun to elucidate the separate distributions of Cope's and Gray Treefrogs. The abstract of McAlpine et al. (1991) makes no reference to the method used to identify Gray Treefrogs, although it is nearly certain that the New Brunswick and Maine Gray Treefrogs are indeed Gray Treefrogs.
Historical versus Current Abundance - Little data comparing historical and current abundance are available. With the advent of the North American Amphibian Monitoring Program [NAAMP]) and its standardized protocols, this paucity of data should be resolved. Most of the early reports of members of this complex suggest, at least qualitatively, that gray treefrogs were common historically. Dickerson (1906) claims that gray treefrogs are second only to American toads (Bufo americanus) in local abundance. Cope (1889, probably D. versicolor in Pennsylvania), LeConte (1825; in Wright, 1932; probably D. versicolor in New York), and Holbrook (1842; in Wright, 1932) refer to gray treefrogs as common. Personal experience in the south suggests that both species are still common. Dense patches occur around ponds in Alabama, but diffuse "populations" of calling Cope's Gray Treefrogs are found in suburban habitats.
Life History Features - Ritke et al. (1990, 1991a) discussed the life history of a western Tennessee population of Cope's Gray Treefrogs.
Breeding - Reproduction is aquatic.
Breeding migrations - Calling is probably stimulated by a combination of day length and temperature. Calling typically begins earlier in the southern than in the northern portions of the species range. Wright (1932) reports gray treefrogs (almost certainly Cope's Gray Treefrogs) from the Okefenokee Swamp calling from 10 June–13 August. In northeastern Alabama, calling can begin in late March and continue through July, but calling is most intense in April–May. In north-central Oklahoma, calling usually begins in April, with a sharp peak in May and early June. Calling usually does not extend very far into July in Oklahoma. Fellers (1997b) reports calling activity in Maryland from April–July or August.
Cope's Gray Treefrogs breed from mid March to July (Wright and Wright, 1949). Wright and Wright (1942) report breeding in Ithaca, New York, (most probably Gray Treefrogs) from April–July. It seems reasonable to suggest that the peak of the breeding season for both species is late spring (May–June).
Breeding habitat - During the breeding season, Gray Treefrogs are found calling near the edges of ponds, ephemeral wetlands and ditches, and from floating algae and emergent vegetation (Fellers, 1979b; Godwin and Roble, 1983; Conant and Collins, 1998; personal observations). At dusk, gray treefrogs may begin calling from high in the trees surrounding a pond. As the evening progresses, individuals move down the trees (sometimes calling along the way) until they reach lower branches or shrubs, or they continue until they reach the ground and move to a point usually within 1.5 m of the water’s edge (personal observations). Godwin and Roble (1983) suggest that females may mate on the first day they arrive at the breeding pond. Both males and females have been observed to mate ≤ 3 times/breeding season (Godwin and Roble, 1983). Ritke and Lessman (1994) present biopsy data that indicate that females have developing follicles throughout the breeding season, and production of later follicles is a function of foraging success. Ritke et al (1991b) report strong breeding pond philopatry.

Egg deposition sites - Eggs are deposited in shallow ponds and pools (permanent or temporary), which may be natural or artificial, pristine or disturbed. Eggs are loosely attached to emergent vegetation at the surface. Runoff and splash at the base of a waterslide enticed calling males, while a nearby dilapidated swimming pool supported breeding in Gray Treefrogs near Kimerling City, Missouri (personal observations).
Clutch size - Eggs are laid in packets (10 x 12.5 cm, 30–40 eggs/packet) as a surface film loosely attached to emergent vegetation (Wright, 1932). Egg number ~1,800 (Wright, 1932).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Film
- Arrangement 2 - Eggs oviposited as adherent films, individual eggs not easily removed from film, top of outer jelly lies at water surface; jelly diameter not large relative to ovum size. Film diameter less than 150 mm, ova pale to dark brown.
- Sub-arrangement B - Eggs deposited in ephemeral nonflowing water; Ovum Diameter 0.8-1.2 mm; 2 jelly layers.
- Arrangement 2 - Eggs oviposited as adherent films, individual eggs not easily removed from film, top of outer jelly lies at water surface; jelly diameter not large relative to ovum size. Film diameter less than 150 mm, ova pale to dark brown.
Tadpoles:

| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |

Larvae/Metamorphosis - Larval length near metamorphosis is approximately 50 mm (Wright and Wright, 1949). Cope (1889) presented drawings of developing larvae of Gray Treefrogs (Plate 78, Figs. 23–26). Thibaudeaux and Altig (1998) documented the ontogenic development of the oral apparatus of Cope's Gray Treefrogs.
Length of larval stage - Dickerson (1906) reported a larval period of 3 wk from eggs to hatching and 4 wk to metamorphosis. Wright (1932) reported 45–65 d for eggs to metamorphosis. Because developmental rates are generally positively linked to temperature, average developmental times are shorter in the South.
Larval requirements - McDiarmid and Altig (1999) have summarized most of what has been published on tadpoles.
Food - Little data are available on the food habits of Cope's Gray Treefrogs or Gray Treefrogs. Tadpoles generally feed by filtering food from the water column or by scraping periphyton from submerged substrates (Hoff et al., 1999). Steinwascher and Travis (1983) reported that Cope's Gray Treefrog tadpoles grew faster on diets with high protein to carbohydrate ratios.
Cover - There are no published reports of cover requirements for Gray Treefrog tadpoles. During the day, tadpoles can be seen resting on a variety of substrates including exposed sediment, leaf litter, and fallen tree limbs. I have observed hylid tadpoles resting on top of these same substrates at night.
Larval polymorphisms - McCollum and Van Buskirk (1996) reported that reddish and yellowish tail pigments are induced by the presence of odonate naiad predators.
Features of metamorphosis - Transformation of tadpoles occurs from late June to August, between 45–65 d post hatching (Wright and Wright, 1949). Larval Cope's Gray Treefrogs are approximately 16 mm TL at metamorphosis (Wright, 1932; Wright and Wright, 1932, 1949).
Post-metamorphic migrations - Roble (1979) describes post-metamorphic migrations of Gray Treefrogs from central Wisconsin. Within a week, juveniles dispersed from their natal ponds. Juveniles moved an average of 1.58 m/d, with maximum dispersal distancess approaching 125 m (Roble, 1979). Juveniles were active throughout the day from July–September.
Juvenile Habitat - Roble (1979) reported that juvenile Gray Treefrogs from Wisconsin were captured on sedges (Carex sp.) about 1/3 of the time, with false nettle (Boehmeria cylindrica), reed grass (Phalaris arundinacea), and swamp white oak (Quercus bicolor) saplings as preferred habitat. Nearly 3/4 (70.3%) of all captures were below 50 cm above the forest floor, while > 2% were found above 1.2 m. Roble (1979) surmised that young frogs did not ascend into trees during their first season.
Adult Habitat - Outside of the breeding season, Gray Treefrogs are found in trees or on mossy or lichen-covered fences, usually above ground (Wright and Wright, 1949; Conant and Collins, 1998), and have been found using abandoned bird houses for shelter (Murphy, 1968). Gray Treefrogs will use introduced tree species such as Chinese tallowtrees (Triadica sebifera) as microhabitat (Fontenot, 2003).
Home Range Size - Little information is known regarding movements outside the breeding season. Adults are thought to spend the remaining part of the activity season high in trees where they forage on insects and insect larvae. Short term movements are probably limited, but during dry seasons, low relative humidity my drive treefrogs to seek out high relative humidity microhabitats.
Territories - Little is known about territoriality in frogs. Gray Treefrogs are known to produce specialized calls (called "turkey roots" by Wright, 1932) when approached while calling. Fellers (1979a) describes territorial behavior in treefrogs. Wells and Taigen (1986) note that call duration increases in high density (mean distance between individuals ~1 m) populations of Gray Treefrogs. Presumably this is a response to intrusion in a territory. Gray Treefrogs will also call prior to thunderstorms. These "rain calls" are common and produced by many hylids (C.M. Bogert, 1960; M. Stewart, personal communication).
Aestivation/Avoiding Dessication - There are no published reports of aestivation in Gray Treefrogs.
Seasonal Migrations - The only migrations reported for Gray Treefrogs are those to the breeding ponds from March–June. In July, individuals may call during periods of high humidity or after rains, but populations tend to be diffuse. Newly metamorphosed Gray Treefrogs move up to 800 m (0.5 mi) from the breeding ponds (Roble, 1979). Dispersal to winter hibernacula have not been described, but such movements are probably short and asynchronous.
Torpor (Hibernation) - Wright (1932) speculated that gray treefrogs remained active in Georgia "until November at least." Harlan (1835, in Wright, 1932) relates collecting a specimen several feet below the surface of the ground from under a root of an apple tree in winter.
Freeze tolerance has been described for Gray Treefrogs. Schmid (1982) reported that glycerol production in Gray Treefrogs allowed individuals to survive -6 ˚C for 5–7 d. Storey and Storey (1985, 1986) reported additional production of glucose as a cryoprotectant. Gray Treefrogs were able to survive -2 ˚C for 5 d and were also capable of surviving repeated freezing and thawing events. Storey and Storey (1986) found Gray Treefrogs could survive moderate freezing temperatures (-2 to -4 ˚C) for ≤ 2 wk. In a laboratory setting, Layne (1991) found that contact with external ice formation triggers freezing in Gray Treefrogs.
Interspecific Associations/Exclusions - While all authors agree that Gray Treefrogs arose from Cope's Gray Treefrogs, there has been considerable disagreement over the possibility of multiple origins for the tetraploid. Maxson et al. (1977) reported immunological hybrids between D. chrysoscelis and D. versicolor in southeastern Oklahoma. Ralin et al. (1983) reported on electrophoretic hybrids from Illinois and argued for a single origin of the tetraploid. However, Ptacek et al. (1994) identified three different D. versicolor lineages based on cytochrome b sequencing. Later, Gerhardt et al. (1994) cytologically confirmed the natural occurrence of a natural triploid hybrids and argued for strong selection against these hybrids in the field. Most recently, Wiley and Little (2000) demonstrated multiple D. versicolor lineages based on the position of the nuclear organizing region and replication banding patterns.
Petranka (1989b) reported possible chemical inhibition of Coastal Plains Leopard Frog (Lithobates sphenocephalus utricularius) (formerly known as Southern Leopard Frog (R. sphenocephala)) tadpoles by Cope's Gray Treefrog tadpoles. Alford and Wilbur (1985) and Morin (1987) reported that both Gray Treefrogs and Cope's Gray Treefrog tadpoles inhibit the presence of other tadpoles, and the order in which species appeared in a pond influenced the community composition at that pond. Ralin (1981) demonstrated that both Cope's and Gray Treefrogs from Texas had similar abilities to respond to desiccation in the same habitats. He further noted greater variability among populations of the same species than he saw between the species.
Age/Size at Reproductive Maturity - Gray Treefrogs range in size from 32–60 mm (Wright and Wright, 1949; Conant and Collins, 1998). Wright (1932) suggests that Gray Treefrogs from the Okefenokee Swamp began breeding at 2 yr of age.
Longevity - Snider and Bowler (1992) report a wild-caught animal lived 7 yr, 9 mo, and 20 d in captivity.
Feeding Behavior - General reports list insects as the prey of Gray Treefrogs (Holbrook, 1842, in Wright, 1932). Puckette (1962) listed roaches, earthworms, and other invertebrates as the major diet items for Gray Treefrogs, but also noted the presence of small snakes. Ralin (1968) reported diet partitioning between diploids and tetraploids: Gray Treefrogs consumed more terrestrial insects, while Cope's Gray Treefrogs ate more arboreal insects. Dickerson (1906) and Ritke and Babb (1991) found Gray Treefrogs to be "sit-and-wait" predators, consuming caterpillars, beetles, flies, wood roaches (Parcoblatta sp., Ischnoptera deropeltiformis), and camel crickets (Ceuthophilus sp.).
Predators - Numerous potential predators exist for hylids, ranging from invertebrates through vertebrates. Dickerson (1906) indicated that diving beetles preyed upon Gray Treefrog tadpoles. Resetarits (1998) reported that odonate naiads and larval dytiscids (diving beetles) both preyed on Cope's Gray Treefrog tadpoles, but larval dytiscids were major egg predators as well. Dragonfly naiads were also used in experimental studies of tadpole response to predators (McCollum and van Buskirk, 1996). A wide variety of fish could prey upon all life stages of Gray Treefrogs. Smith et al. (1999) reported that bluegill sunfish (Lepomis macrochirus) significantly reduced Gray Treefrog tadpole abundance in field experiments. Potential amphibian predators include salamanders and salamander larvae (chiefly Notophthalmus sp. and Ambystoma sp.) and some adult frogs (i.e., American Bullfrog (Lithobates catesbeianus)). Skelly (1992) used tiger salamanders (Ambystoma tigrinum) in field experiments of antipredator costs. Numerous turtles and snakes represent potential predators on the various stages of the life cycle of frogs. Wading birds, especially herons (Ardeidae), prey upon tadpoles and frogs of many species. Additionally, raccoons (Procyon lotor) and striped skunks (Mephitis mephitis) are potential mammalian predators.
Anti-Predator Mechanisms - Banta and Carl (1967) noted the occurrence of "death-feigning" behaviors in male frogs following handling or capture. Brodie and Formanowicz (1981b) noted that Gray Treefrogs are apparently unpalatable to shrews (Blarina brevicauda), which may be due to noxious skin secretions. Mucus secretions are foul tasting and cause burning sensation and inflammation in the mucus membranes of the eyes (personal observation). While these secretions have antipredator functions, it is also possible that they also function as antimicrobial agents.
Tadpoles may not use chemical defense compounds. Kats et al. (1988) categorized Cope's Gray Treefrog tadpoles as "palatable" in their predation studies. Tadpole morphology has a significant influence on predator escape. Van Buskirk and McCollum (1999) observed that Gray Treefrog tadpoles from predator-rich ponds tended towards having longer, shallower bodies, and shallow, brightly pigmented tails. Following up that observation, van Buskirk and McCollum (2000a) found that these tadpoles accelerated faster than other shapes. Further, van Buskirk and McCollum (2000b) demonstrated that 30% of the tail must be removed before swimming performance is affected. These studies suggest that tadpoles distribute pigments in the tail to misdirect predator attacks. Several studies have also reported decreased tadpole activity (Lawler, 1989) and a shift in habitat usage (Formanowicz and Bobka, 1989) in the presence of predators. Resetarits and Wilbur (1989) concluded that Cope's Gray Treefrog tadpoles were capable of detecting chemical odors of potential predators in predator conditioned water.
Diseases - Emerging infectious diseases have been increasingly implicated in the local extinction of many amphibian populations worldwide. Chytrid fungi and ranaviruses have been implicated in local extinctions in ranid, bufonid, pelodryadid treefrogs, newt, and ambystomatid populations worldwide (Daszak et al., 1999). So far, no known reports of hylid frog declines are related to chytrid fungi or ranaviruses.
Parasites - A variety of parasite hosts have been suggested for Cope's and Gray Treefrogs. Woo and Bogart (1984) reported two species of trypanosomes from Cope's and Gray Treefrogs. Delvinquier and Dresser (1996) reported an opalinid (Sarcomastigophora) from Gray Treefrogs. Hausfater et al. (1990) chose Gray Treefrogs as a model organism for studying the effect of parasitism on mate choice, in part because males harbored a “wide range of helminth parasites."
Conservation - McAlpine et al. (1991) suggested that the range of Gray Treefrogs is expanding in Maine and New Brunswick as the result of human activity. Bunnell and Zampella (1999) report similar conclusions for Gray Treefrogs from the border of the New Jersey Pine Barrens. Lannoo et al. (1994) report that Gray Treefrogs have persisted in an Iowa county 70 yr since an earlier report. McAlpine et al. (1991) indicated that Gray Treefrogs from New Brunswick and Maine were neither rare nor endangered. Kolozsvary and Swihart (1999) consider Gray Treefrogs ubiquitous in Indiana and not affected by habitat patch size. This disagrees with Hager (1998), who concludes that Gray Treefrogs are sensitive to patch size because they were excluded from smaller islands in Lake Erie, Georgian Bay, and the St. Lawrence River.
Several studies (Karns, 1992; Grant and Licht, 1993; Pehek, 1995) indicate that Gray Treefrogs are at least moderately tolerant of pH levels as low as 3.5. Ambient levels of UV-B radiation had no negative effects on development or survival of eggs and larvae of Gray Treefrogs from Ontario (Grant and Licht, 1995). Zaga et al. (1998) reported a negative effect of UV-B radiation on swimming activity of Gray Treefrogs. Additionally, Zaga et al. (1998) reported no significant effect of the carbamate pesticide, carbaryl, but they did find that UV-B radiation photoenhanced the toxicity of carbaryl as measured by swimming activity. The pesticide carbaryl had no significant effect on activity in Gray Treefrogs tadpoles (Bridges, 1999b; Zaga et al., 1998).
The overall impression is that Gray Treefrogs are moderately tolerant to the pollutants tested so far, and that they are tolerant to human habitat disturbance.
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Albemarle
Alleghany
Amherst
Appomattox
Augusta
Bath
Bedford
Bland
Botetourt
Brunswick
Buckingham
Campbell
Charles City
Charlotte
Chesterfield
Clarke
Craig
Culpeper
Cumberland
Fairfax
Fauquier
Franklin
Frederick
Giles
Goochland
Greene
Halifax
Henrico
Henry
Highland
King George
King William
Loudoun
Louisa
Madison
Mecklenburg
Montgomery
Nelson
Nottoway
Orange
Page
Patrick
Pittsylvania
Powhatan
Prince Edward
Prince William
Pulaski
Rappahannock
Rockbridge
Rockingham
Shenandoah
Smyth
Spotsylvania
Stafford
Sussex
Warren
CITIES
Charlottesville
Harrisonburg
Lynchburg
Radford
Virginia Beach
Verified in 56 counties and 5 cities.
U.S. Range