Southern Chorus Frog
Pseudacris nigrita
Common Name: |
Southern Chorus Frog |
Scientific Name: |
Pseudacris nigrita |
Etymology: |
|
Genus: |
Pseudacris is derived from the Greek words pseudes meaning "false" and akris meaning "locust". |
Species: |
nigritais Latin meaning "blackened" |
Average Length: |
0.8 - 1.3 in. (1.9 - 3.2 cm) |
Virginia Record Length: |
|
Record length: |
Virginia Wildlife Action Plan Rating Tier IV (Moderate Conservation Need) - The species may be rare in parts of its range, particularly on the periphery. Populations of these species have demonstrated a significant declining trend or one is suspected which, if continued, is likely to qualify this species for a higher tier in the foreseeable future. Long-term planning is necessary to stabilize or increase populations.
Physical Description - A small light tan or gray frog with small toe pads and a dark brown stripe extending from the snout down each side. Adult snout to vent length is 0.75 to1.25in (1.9-3.2cm). A white line runs along the lip. On the back are usually three rows of broken stripes or blotches. Belly is whitish. Although this species is primarily nocturnal, it occasionally is found during the daytime. Because the call and appearance is very similar to the Upland Chorus Frog (Pseudacris feriarum), the Southern Chorus Frog is often misidentified as an Upland Chorus Frog. They breed from late winter to early spring.
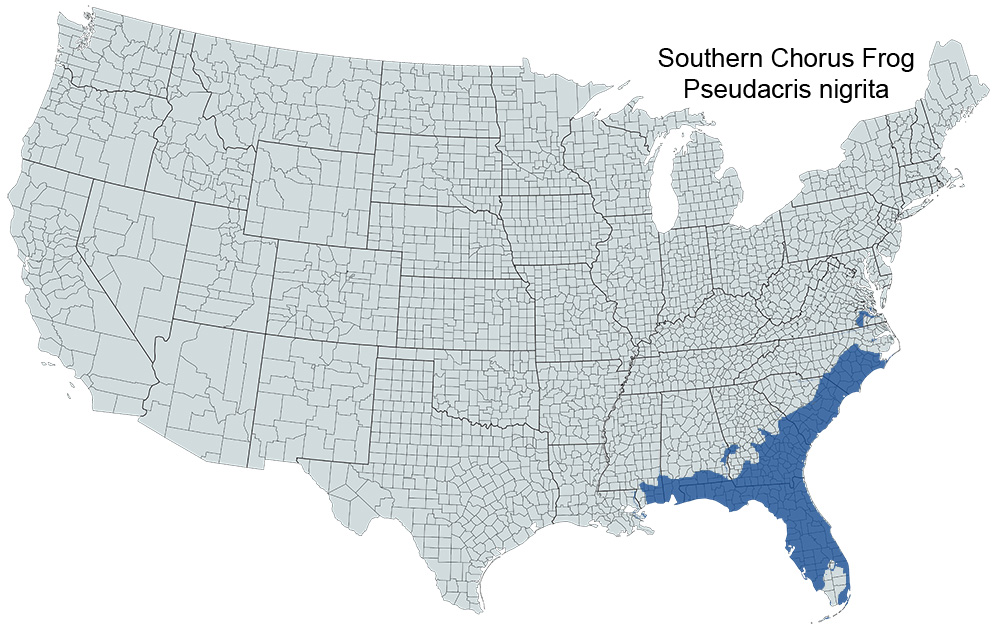
Historical versus Current Distribution - Southern Chorus Frogs (Pseudacris nigrita) inhabit the Gulf Coastal Plain from just north of the Tar and Pamlico rivers in North Carolina to the Pearl River in southern Mississippi (Schwartz, 1957a; Gates, 1988). They are absent from the Everglades and the Florida Keys (Stevenson, 1976). There is no evidence to indicate any change in distribution over the historical period.
Historical versus Current Abundance - Common to abundant in Georgia (Martof, 1956a). Abundant in Harrison County, Mississippi, through the winter and early spring (Allen, 1932). Not common in Florida (Carr, 1940a; Wright and Wright, 1949). Moderately common in Alachua County, Florida (Van Hyning, 1933). There is no evidence in the literature to indicate a change in abundance of this species over time. However, longleaf pine flatwoods, habitat in which Southern chorus frogs are a characteristic species (Carr, 1940a), have been reduced drastically by conversion to rapidly cropped slash pine plantations throughout the Gulf Coastal Plain (Means et al., 1996; Means, 2003a). Ponds within these flatwoods have been connected by ditches in order to drain them more rapidly after rains (Vickers et al., 1985), and fire has been suppressed in the plantations (Enge and Marion, 1986). It is likely that the abundance and distribution of amphibians inhabiting these flatwoods generally have been affected by these changes. Enge and Marion (1986) compared amphibian species richness and abundance in a naturally regenerated 40-yr-old slash pine forest to recent clearcuts within the forest. They concluded that clearcutting reduced amphibian abundance tenfold by affecting reproductive success but did not affect species richness. However, a comparison has not been made of current amphibian species abundance and distribution in these plantations to a pine flatwoods forest where fire has not been suppressed and that has not been cut and ditched. The time for such a comparison may have passed, as only about 0.01% of old-growth longleaf pine forest remains (Means et al., 1996; Means, 2003a).
Breeding - Reproduction is aquatic.
Breeding migrations - Breeding migrations occur almost exclusively at night (Pechmann and Semlitsch, 1986).
Breeding habitat - Southern Chorus Frogs breed in temporary pools, roadside ditches, woodland ponds, and Carolina bays (Caldwell, 1987); flooded fields, roadside ditches, the weedy margins of shallow flatwoods ponds, and temporary woodland pools (Mount, 1975); ditches, bogs, and shallow ponds (Martof et al., 1980); flooded pine-wood ditches, borrow pits, gum ponds in pine woods, and flooded fields surrounded by pine woods (Schwartz, 1957a). Southern Chorus Frogs breed in wet seasons in otherwise xeric habitats (Schwartz, 1957a; Duellman and Schwartz, 1958).
Southern Chorus Frog males are secretive and generally call from the bases of grass tussocks and under overhanging grass on the edges of ponds, with only their heads protruding above the water (Einem and Ober, 1956; Mount, 1975; Gartside, 1980). Males call from locations where the vegetation is most dense. Ornate Chorus Frogs (Pseudacris ornata) call from open, exposed situations at the same localities (Schwartz, 1957a). In southern Florida, males call from within clumps of grasses, from the ground, or from holes and cracks in the limestone borders of sinkholes (Duellman and Schwartz, 1958). All members of the nigrita species group are basically similar in that the males call just out of the water or (most often) partially immersed, or even floating in the water with the forelimbs supported by emergent stems or twigs (Mecham, 1965).
In southwestern Georgia, breeding sites of southern Chorus Frogs are widely scattered in the uplands, whereas Upland Chorus Frogs breed in floodplain pools in terrain more similar to that of the Piedmont to the north (Crenshaw and Blair, 1959).
In South Carolina and Alabama, the breeding season begins as early as December and extends well into April and possibly into May (Mount, 1975; Gibbons and Semlitsch, 1991). Heavy thundershower activity may initiate vigorous calling during summer or even fall, but breeding activity at this time is unlikely (Mount, 1975). In contrast, southern Chorus Frogs in southern Florida breed from January–September, with peak activity in June and July (Duellman and Schwartz, 1958). Southern Chorus Frogs are considered prolonged breeders (Caldwell, 1987).
Egg deposition sites - Females deposit small, irregular egg clusters of about 15 eggs each on stems, leaves, or other objects in shallow water (Martof and Thompson, 1958; Martof et al., 1980), but sometimes deposit on the bottom of shallow pools (Brandt, 1953).
Clutch size - About 2 hr are required to lay a total of 78–157 eggs (Martof and Thompson, 1958). In southern Florida, a female laid 160 eggs, a few separately but the majority in a single loose mass (Brady and Harper, 1935). Hatching took place within 60 hr in January in southern Florida (Brady and Harper, 1935).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Mass
- Arrangement 3 - Capsular chambers absent, so ova centered in inner jelly layers mass usually 45 mm diameter or less (note: particularly difficult group with minimal data).
- Sub-arrangement B - Ovum Diameter 0.9-1.0 mm; Egg Diameter 2.6-2.8 mm; 1 jelly layer.
- Arrangement 3 - Capsular chambers absent, so ova centered in inner jelly layers mass usually 45 mm diameter or less (note: particularly difficult group with minimal data).
Larvae/Metamorphosis - The larval period lasts from about 40–60 d (Wright and Wright, 1949; Martof et al., 1980) to about 4 mo (Caldwell, 1987). Juveniles metamorphose at 9–15 mm SVL (Wright and Wright, 1949).
Tadpoles:

| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |

Juvenile Habitat - Juveniles often remain for several weeks near the ponds from which they emerged (Carr, 1940a).
Adult Habitat - Southern Chorus Frogs are considered pine savanna (Martof et al., 1980) or pine flatwoods species (Carr and Goin, 1959). In Alabama, where they are sympatric with Upland Chorus Frogs, southern Chorus Frogs are likely to be found where the soil is sandy and friable; Upland Chorus Frogs occur mostly on places where the soil is heavier (Mount, 1975). In southern Florida, Southern Chorus Frogs are always associated with limestone sinkholes, especially bordering wet prairies, but are absent from wet prairies proper and from sandy country (Duellman and Schwartz, 1958). Adults move to drier hammocks and ridges of the Pine Barrens (Wright and Wright, 1949).
Home Range Size - Unknown.
Territories - Unknown.
Aestivation/Avoiding Dessication - Carr (1940a) found that captive individuals almost invariably burrow deep in the soil of their cages after a few days of confinement and suggested that the adults may lead an almost wholly subterranean existence. Because these frogs prefer xeric habitats that are only occasionally inundated (Schwartz, 1957a; Duellman and Schwartz, 1958), they may spend time underground in aestivation.
Seasonal Migrations - Unknown.
Torpor (Hibernation) - Unknown.
Interspecific Associations/Exclusions - In southwestern Georgia, Southern Chorus Frogs and Upland Chorus Frogs inhabit the Upper Coastal Plain of North Carolina, but no hybridization occurs, even when they breed in the same locality (Batts, 1960). However, Gartside and Dessauer (1976) found a narrow (10–20 km wide) zone of hybridization along the Pearl River, which forms the southern border between Louisiana and Mississippi. In an area of sympatry along the Georgia–Alabama border, Fouquette (1975) observed character displacement in the breeding calls (both pulse rate and number of pulses) that resulted in reproductive isolation. To the north and south of this area of sympatry, the difference in pulse rate and number was reduced to a degree probably not sufficient for effective female discrimination of male calls.
Age/Size at Reproductive Maturity - Age at first reproduction 12–14 mo, including the approximately 4-mo-long tadpole stage (Caldwell, 1987; Gibbons and Semlitsch, 1991). Adult size is 25–33 mm SVL (Caldwell, 1987).
Longevity - The population turnover is nearly annual. A few individuals may live for 2, rarely 3 yr (Caldwell, 1987). Survival of males at a breeding site ranged from 15–34%, while survival of females ranged from 27–55%. The average time spent at the breeding site varied from 4–25 d, depending on the year. The highest survival rates occurred in years when less time was spent at the breeding site (Caldwell, 1987).
Feeding Behavior - Examination of the stomach contents of ten individuals revealed the remains of ants and small beetles (Duellman and Schwartz, 1958). Carr (1940a) watched adults and recently emerged young catching grasshopper nymphs in short grass at the edge of ponds. He commented that their feeding behavior is similar to that of cricket frogs (Acris sp.).
Predators - Salamanders, particularly tiger salamander (Ambystoma tigrinum) larvae, and aquatic insects are likely predators of Southern Chorus Frog tadpoles (Caldwell, 1987).
Anti-Predator Mechanisms - Breeding migrations of southern Chorus Frogs occur almost exclusively at night, probably to reduce predation risk from visual predators, particularly from diurnal birds such as crows and shrikes (Pechmann and Semlitsch, 1986). The presence of grasses in a shallow water body indicates that it has been dry in recent months and is likely to be relatively free of predators. Grassy calling sites, preferred by southern Chorus Frog males, may thus constitute a proximate cue indicating a relatively predator-free larval environment (Caldwell, 1987).
Diseases - Unknown.
Parasites - Unknown.
Conservation - Southern Chorus Frogs are not protected either by state or federal laws. There are no data to indicate any change in distribution, however, their native longleaf pine flatwood habitat has been drastically reduced (only 0.01% of old-growth longleaf pine forest remains) by (1) conversion to slash pine plantations, (2) connecting ponds for drainage, and (3) fire suppression. In the face of these activities, it is likely that Southern Chorus Frogs have experienced population reductions and extirpations.
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Prince George
Southampton
Surry
Sussex
York
CITIES
Newport News
Verified in 5 counties and 1 cities.
U.S. Range